- The students study were discussing the mass number, which is a way of describing the massive. And Adam Now on Adam consists of an exact number of protons, neutrons and electrons. But the mass number, which it can also be abbreviated as a, is exactly the number of protons and the number of neutrons oven adam.
- The number of nucleons present in an atom is called mass number. Mass number, as from the name, is responsible for the mass of an atom or atomic mass. Since protons and neutrons are much heavier than electrons, the mass of an atom is estimated by the number of protons and the number of neutrons or in other words, mass number.
- Mass Number Equals Protons plus Neutrons Each atom also has a mass number, denoted by the symbol A. The mass number of an atom is equal to the number of protons plus the number of neutrons that it contains. In other words, the number of neutrons in any atom is its mass number minus its atomic number.
Contents
Atomic number, chemical symbol, and mass number Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Its average atomic mass is 12.11. Scientists determine the atomic mass by calculating the mean of the mass numbers for its naturally-occurring isotopes.
- Atomic number and Mass number
- Isotopes
Atom
An atom is the smallest particle of an element which can take part in chemical reaction. Atom consists of three fundamental particles i.e. proton, neutron and electron. Atoms of same elements are similar in properties whereas atoms of different elements are different in properties. Example:- ‘H’ represent the atom of hydrogen.
Proton is positively charged and electron is negatively charged particle. In an atom, number of protons = number of electrons. Hence, the net charge present in an atom is zero i.e. a free atom is chargeless.
Atomic number and Mass number
Atomic number :
- Atomic number is the number of protons present in an atom.
- The modern periodic table is arranged in order of increasing atomic number.
Mass number and Atomic mass :
- Mass number is the sum of the number of protons and the number of neutrons present in an atom. It is a whole number.
Mass no. of an atom = No. of protons + No. of neutrons
- Atomic mass is the average mass of the all of the isotopes of that element. It is a decimal number.
- For example: Hydrogen has three isotopes – 1H1, 1H2 and 1H3 having mass number 1, 2 and 3 respectively. Naturally occurring hydrogen contains about 99.985% of protium, 0.014% of deuterium and 0.001 % of tritium. Therefore the atomic mass of hydrogen is 1.00784 amu.
- The atomic mass of an element element is measured in atomic mass unit (amu, also known as Daltons ‘ D’or unified atomic mass unit ‘u’).
- 1amu = 1.66 x 10-24 grams. 1gm = 6.022 x 1023 amu ( i.e. Avogadro’s number).
Here,
- Atomic number = Number of protons = Number of electrons = 13
- Mass number = No. of protons + No. of neutrons
- No. of neutrons = Mass number – No. of protons = 27-13 = 14.
Atomic mass of first 20 elements
| Atomic number | Element | Atomic mass |
| 1 | Hydrogen | 1.008 |
| 2 | Helium | 4.0026 |
| 3 | Lithium | 6.94 |
| 4 | Beryllium | 9.0122 |
| 5 | Boron | 10.81 |
| 6 | Carbon | 12.011 |
| 7 | Nitrogen | 14.007 |
| 8 | Oxygen | 15.999 |
| 9 | Fluorine | 18.998 |
| 10 | Neon | 20.180 |
| 11 | Sodium | 22.990 |
| 12 | Magnesium | 24.305 |
| 13 | Aluminium | 26.982 |
| 14 | Silicon | 28.085 |
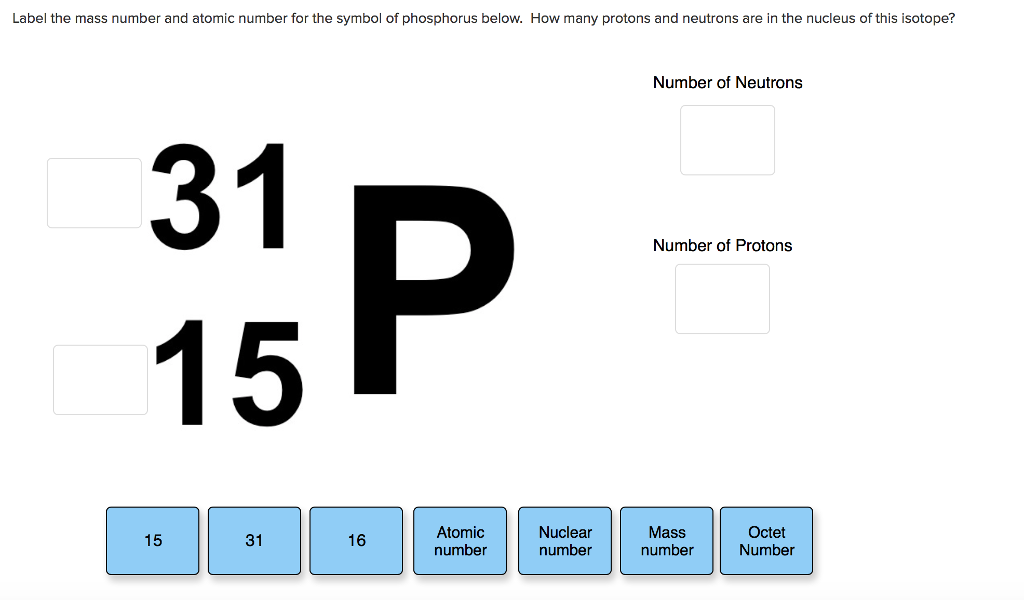
| 15 | Phosphorus | 30.974 |
| 16 | Sulfur | 32.06 |
| 17 | Chlorine | 35.45 |
| 18 | Argon | 39.948 |
| 19 | Potassium | 39.098 |
| 20 | Calcium | 40.078 |
Isotopes
Atoms of the same element having same atomic number but different mass number (atomic mass/weight) are called isotopes. For example:
Isotopes of hydrogen :
There are three isotopes of hydrogen:
- Protium or ordinary hydrogen
- Deuterium or heavy hydrogen
- Tritium or radioactive hydrogen.
| Name | Protium | Deuterium | Tritium |
| Symbol | 1H or H | 2H or D | 3H or T |
| No. of protons(P) | 1 | 1 | 1 |
| No. of neutrons(n) | 0 | 1 | 2 |
| No. of electrons(e) | 1 | 1 | 1 |
| Atomic no.(Z) | 1 | 1 | 1 |
| Mass no.(A) | 1 | 2 | 3 |
Naturally occurring hydrogen contains about 99.985% of protium, 0.014% of deuterium and 0.001 % of tritium.
Isotopes have different physical properties since they differ in their mass number.
They have same chemical properties since their electronic configuration is same. However, they differ in the rate of chemical reaction. For example, D2 reacts with Cl2 about 13 times slower than H2 does. The different in rate of reaction due to difference in mass of the atoms of the same element is called isotope effect.
Some other examples of isotopic elements :
| Elements | Isotopes | Most abundant isotope |
| Carbon | 6C12, 6C13, 6C14 | 6C12 |
| Nitrogen | 7N14, 7N15 | 7N14 |
| Oxygen | 8O16, 8O17, 8O18 | 8O16 |
| Sulphur | 16S32, 16S33, 16S34, 16S36 | 16S32 |
| Chlorine | 17Cl35, 17S37 | 17Cl35 |
Isobars
Atoms of different elements having different atomic number but same mass number are called isobars. For example :
18Ar40, 19K40 and 20Ca40

Isotones
Atoms of different elements having different atomic number and mass number but same number of neutrons are called isotones. For example :
6C14, 7N15 and 8O16
Objective questions and their answers
1. Which of the following is known as heavy hydrogen?
a. Protium c. Tritium
b. Deuterium d. Para hydrogen
2. Which of the following is known as radioactive hydrogen?
a. Protium c. Tritium
b. Deuterium d. Para hydrogen
3. Least abundant isotope of hydrogen is:
a. Protium c. Tritium
b. Deuterium d. Heavy hydrogen
4. Diamond and graphite are :
a. Isotopes c. Isotones
b. Isobars d. Allotropes
5. 6C14 and 8O16 are :
a. Isotopes c. Isotones
b. Isobars d. Allotropes
6. 6C14 and 7N14 are :
a. Isotopes c. Isotones
b. Isobars d. Allotropes
7. All particles residing inside the nucleus of an atom are termed as:
a. Protons c. Electrons
b. Neutrons d. Nucleons
8. What makes the atomic mass fractional ?
a.Prerence of isotopes
b. Number of unpaired electrons
c. Spherical shape
d. Quantum number.
Logitech c270 mac software download. 9. Which of the following are not isotopes: Cubase 10.5 dc.
a. 1H1 and 1H3
b. 18K40 and 20Ca40
c. 6C14 and 7N14 Adobe illustrator mac download.
d. Both b and c.
10. Charge present in the nucleus of an atom is :
a. Positive c. Chargeless
b. Negative d. Both +Ve and -Ve
11. Molecular weight of heavy water is :
a. 16 c. 20
b. 18 d. 22
Answers :
1. b 2. c 3. c
4. d [Note : different forms of same element having different properties are called allotropes]
5. c 6. b 7. d
8. a 9. d 10. a
11. c Note :Heavy water– Deuterium oxide (D2O) is called heavy water. It’s molecular weight is 20 and boiling paint is 101.50C and melting point is 3.80C.
References
- Sthapit, M.K., Pradhananga, R.R., Foundations of Chemistry, Vol 1 and 2, Fourth edition, Taleju Prakashan, 2005.
20th Jun 2019 @ 13 min read
An atom consists of electrons, protons, and neutrons. Protons and neutrons reside in the nucleus of an atom, and they, in together, are called nucleons. The number of nucleons present in an atom is called mass number. Mass number, as from the name, is responsible for the mass of an atom or atomic mass. Since protons and neutrons are much heavier than electrons, the mass of an atom is estimated by the number of protons and the number of neutrons or in other words, mass number. Mass number is always a whole number because the number of protons and neutrons present in an atom is always a whole number.
Definition
Mass number is the number of nucleons (the sum of protons and neutrons) present in an atom. Mass number is also called as atomic number or nucleon number.
The Mass Number Of An Atom Is Obtained By Totaling The Number Of
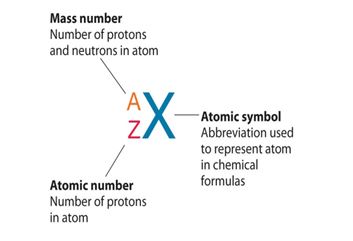
Notation and Formula
Mass number is denoted as A. The value of A is calculated by summing the number of protons and neutron in the nucleus of an atom.
where: np+ is the number of protons and nn0 is the number of neutrons.
Representation
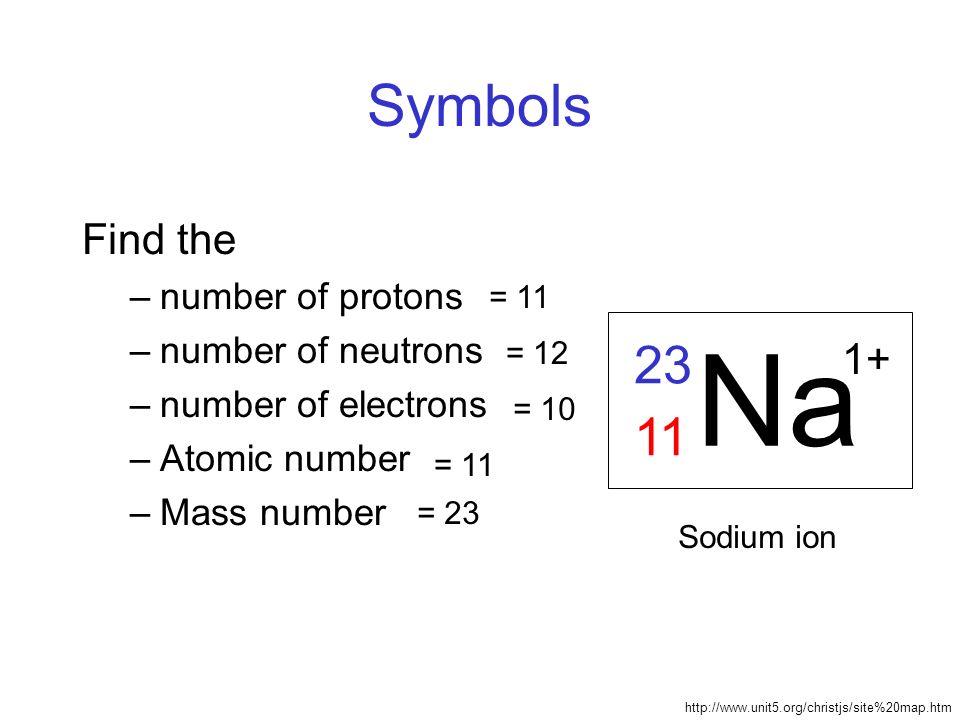
The mass number is represented as the left superscript to the symbol of an element. Consider an example of sodium, which has a mass number of 23. The number is superscripted to the left of the symbol of sodium, which is Na (see the below figure). When using the full name of an element, not its symbol, mass number come after the name of an element with a hyphen in between, for example, sodium-23, carbon-12, iron-56.
Mass Number and Atomic Number
Mass number and atomic number are often confusing for beginners, but they both are different. The below table distinguishes them.
| Mass Number | Atomic Number |
| Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. | Atomic number is the number of protons present in the nucleus of an atom. |
| It is denoted as A. | It is denoted as Z. |
| It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. | It is always fixed for a given element, for example, the atomic number of an oxygen atom is 16. This is true for any oxygen atom in the universe. |
| Two different elements can have the same mass number as in 40 20Ca and 40 18Ar. | Two different elements always have a different atomic number as in 40 20Ca and 40 18Ar. |
| It is always a whole number. | It is also always a whole number. |
| It is always larger than atomic number (except in the case of 1 1H where both are equal). | It is smaller than mass number (except in the case of 1 1H where both are equal). In fact, atomic number is a subset of mass number. |
Mass Number, Atomic Number and Neutron Number
Mass number (A), atomic number (Z), and neutron number (N) relate to each other as simple mathematically equation, which is mentioned below.
Mass Number and Atomic Mass
Mass number is nearly equal to atomic mass of an element. Mass number is a whole number while atomic number occurs in decimals except in the case of carbon-12, which has the exact atomic mass of 12 u. Mass number is a dimensionless quantity while atomic mass is expressed in the atomic mass unit (amu or u). For most of common elements, the difference is very small in the range of (1 u). The difference between them is called mass excess. The table below list some of elements with their mass number and atomic number.
| Element | Atomic Mass, ma in (u) | Mass Number, A | ma∕A |
| 1 1H | 1.007 | 1 | 1.007 |
| 12 12C | 12 | 12 | 1 |
| 23 11Na | 22.990 | 23 | 0.999 56 |
| 35 17Cl | 34.969 | 35 | 0.999 11 |
| 56 26Fe | 55.935 | 56 | 0.998 84 |
| 202 80Hg | 201.971 | 202 | 0.999 856 |
| 235 92U | 235.044 | 235 | 1.000 187 |
| Note: The atomic mass mentioned in the below table is of isotopic elements; it should not be confused with average atomic mass. | |||
As we can observe from the above table, the ratio of atomic mass to mass number is close to one except in the case of carbon, where it exactly equals one.
Mass Number and Isotopes
Mass number is very useful in distinguishing the isotopes of an element. Isotopes are atoms of the same element which have the same atomic number but a different mass number. Consider an example of uranium, which has two naturally occurring isotopes: uranium-235 (235
92U) and uranium-238 (238
92U). Both the isotopes have the same proton number or atomic number. we can differentiate these two by mass number or atomic mass. In fact, we can separate these isotopes with the help of this difference. In uranium enrichment plants, (235
92U), which is fissile, is separated from (238
92U) using centrifugation. Centrifugation is a technique in which the constituents are separated using the difference in their masses.
Mass Number in Nuclear Reactions
Nucleons of an atom do not take part in a chemical reaction, only electrons do. Thus, mass number of an element is unaltered in a chemical reaction. But this is not a case with nuclear reactions. In nuclear reactions, mass number may be or may not be altered. Consider the famous uranium fission reaction.
As we can see, uranium accepts a neutron to form barium and krypton. The mass number of the reactant and the products are different. But in overall reaction the mass number is conserved.
Consider one more example of the natural decay of carbon-14. The carbon-14 is a naturally occurring radioactive isotope, which decays by emitting β-rays. The reaction is as follows:
The Atomic Number Of An Atom Is The Number Of
In the above reaction, carbon-14 decays to nitrogen-14 by transmitting an electron and an antineutrino. There is a change in atomic number, but mass number is the same in both carbon and nitrogen.
Examples
Example 1: Mass Number of Hydrogen
Statement: Hydrogen atom contain only one proton and no neutron. What is the mass number of the hydrogen atom?
Solution: Since there is only one proton and no neutron, the number of nucleons is one. Thus, mass number is also one.
Example 2: Mass Number of Carbon
Statement: The atomic number of carbon is 6. For an atom of carbon, neutron number also equals 6. What is mass number of the carbon atom?
Mass Number Is
Solution: The atomic number (Z) is 6 and neutron number (N) is also 6. The mass number (A) is calculated as:
Example 3: Mass Number of Chlorine
Statement: The atomic number of chlorine is 17. For an atom of chlorine, neutron number equals 18. What is mass number of the chlorine atom?
Solution: The atomic number (Z) is 17 and neutron number (N) is 18. The mass number (A) is calculated as:
Associated Articles
The Mass Number Of An Element Is The Number Of
If you appreciate our work, consider supporting us on ❤️ patreonCarbon Atom Mass Number
.Atom Mass Number Definition
- 5
- cite
- response
Copy Article Cite
