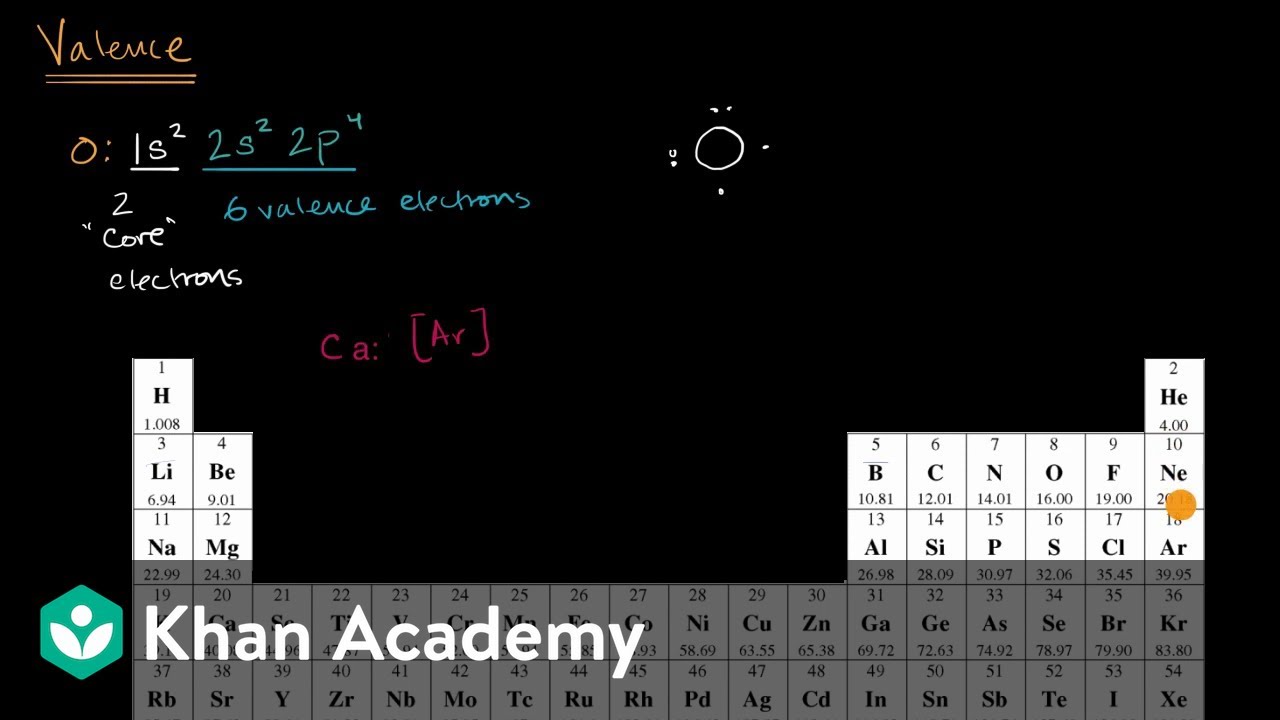
In thermodynamics, mean free path can be calculated in Copper from the atomic weight, valence electrons and density.
Note: Copper have one free electron per atom. Electron mean free path of (Copper) Cu to be 55 nm.
Calculate the Mean Free Path of Electrons in Copper
Conductors and Insulators Conductors. Conductors and Insulators. As was shown in Figure 741.1.2, the nucleus of the copper atom. Contains 29 protons. A neutral copper atom must therefore have 29. Electrons distributed amongst its various shells. Shells k, 1, and mare. Filled to capacity with a total of 28 electrons, so there is only one. Copper also happens to be a transition element and is known to have two valence states: cuperous (1 valence electron) and cupric (2 valence electrons). In the former, copper will lose the 4 s.
Copper with a +3 charge loses the 4-s orbital valence electrons and a 3-d orbital valence electron. Remember that there are 5 3-d orbitals, so having 6 electrons in these 5 orbitals is unstable, so Cu+2 will lose the extra electron in the 3-d. So, there are only the remaining 5 valence electrons in the 3-d orbitals. Copper is a good conductor because, like other metals, it contains free electrons. Free electrons are also known as conduction electrons. Each copper atom provides a single free electron, so there are as many free electrons as atoms. Free electron concentration in copper n = 8.5 × 10 28 per m 3.
In thermodynamics, mean free path can be calculated in Copper from the atomic weight, valence electrons and density.
Note: Copper have one free electron per atom. Electron mean free path of (Copper) Cu to be 55 nm.
Formula:
U = VE * ro / AwEp = 829.4 * UC1 = 53.4 - 20.8 * UC2 = 1.97 - 0.91 * Uγ = 0.191 / ro2β = -0.10 + 0.944 / (√( Ep + BG2 )) + (0.069 * ro2 )λe = 1000 / [ Ep (β × log(γ * 1000) - ( C2 / 1000 ) + (C1 / 10002)) ]Where,λe = Mean Free Path in CopperAw = Atomic WeightVE = Valence ElectronsBG = Bandgap (Copper = 0.0)ro = DensityU = MeanEp = Energy C1, C2 = Radiusβ, γ = Radio Active ChargesRelated Calculators:
Top Calculators
Popular Calculators
Top Categories
| Name: Copper | Boiling Point: 2840°K, 2567°C, 4653°F Melting Point:1357.75°K, 1084.6°C, 1984.3°F Electrons Energy Level: 2, 8, 18, 1 Isotopes: 26 + 2 Stable Heat of Vaporization: 300.3 kJ/mol Heat of Fusion: 13.05 kJ/mol Density: 8.96 g/cm3 @ 300°K Specific Heat: 0.38 J/g°K Atomic Radius:1.57Å Ionic Radius: 0.73Å Electronegativity: 1.9 (Pauling); 1.75 (Allrod Rochow) Vapor Pressure: 0.0505 Pa @ 1084.6°C |
1s2 2s2p6 3s2p6d104s1
|
|
The Egyptians found that adding a small amount of tin made the metal easier to cast, sobronze alloys were found in Egypt almost as soon as copper was found. Copper isfound extensively in the Indus Valley Civilization by the 3rd millennium BC. Use ofcopper in ancient China dates to at least 2000 BC. By 1200 BC excellent bronzes werebeing made in China. Note that these dates are affected by wars and conquest, ascopper is easily melted down and reused. In Europe, Oetzi the Iceman, awell-preserved male dated to 3200 BC, was found with a copper-tipped axe whose metal was99.7% pure. High levels of arsenic in his hair suggest he was involved in coppersmelting. Brass, an alloy of zinc and copper, was known to the Greeks but first usedextensively by the Romans.
Alchemical Symbol, Cuprum
There are copper and bronze artifacts from Sumarian cities that date to 3000 BC, andEgyptian artifacts of copper and copper-tin alloys nearly as old. In one pyramid, acopper plumbing system was found that is 5000 years old. In the Americas productionin the Old Copper Complex, located in present day Michigan and Wisconsin, was dated backto at least 6000 to 3000 BC.
In Greek times, the metal was known by the name chalkos (equivalent of Roman aes)and named after the copper mines at Chalcis in Euboea. This stem is often seen asreferring to copper, notably in mineralogy. 'Chalcopyrite' is copperpyrites. The word was applied to iron as well after its introduction, long beforethe coining of sideros, 'sideros'. The word forsteel is caluy, clearly related to chalcos. Copper was avery important resource for the Romans and Greeks.
Additional Representation of Alchemical Symbols for Copper
In Roman times, it became known as cyprium aes, 'brass from Cyprus' or'brass of Venus' (aes being the generic term for copper alloys such asbronze-brass and other metals, and Cyprium because so much of it was mined inCyprus). From this, the phrase was simplified to cuprum and then eventuallyAnglicized into the English copper. Copper was associated with the goddessAphrodite/Venus in mythology and alchemy, owing to its lustrous beauty, its ancient use inproducing mirrors, and its association with Cyprus, which was sacred to the goddess. The Venusian name stuck in the West, and gave us cobre in Spanish, cuivrein French and Kupfer in German. In Welsh it is copr, which suggeststhat this word comes from old Celtic, and is unassociated with Venus. ThePhoenicians, and others, worked copper mines and smelters in Cyprus, Kupris.Venus, or Aphrodite, the Kupris, was born there, always loved theisland, and was its patron. She is very often referred to as Cypris. The word'cuprium' could have come either from the island or the goddess, and it isimpossible to make a distinction. The word cuprum was probably later Latin,and gave us the chemical symbol Cu. The Phoenicians were surely the ones who spreadthe knowledge and use of copper around the Mediterranean, and their metallurgicalprocedures were probably kept secret. Our word 'copper' comes from thePlattdeutsch coper or koper, still used in Dutch.
In alchemy the symbol for copper was also the symbol for theplanet Venus
Valence Electron Configuration Of Copper
Copper, as native copper, is one of the few metals to naturally occur as anuncompounded mineral. Copper was known to some of the oldest civilizations onrecord, and has a history of use that is at least 10,000 years old. Mosfet small signal. A copper pendantwas found in what is now northern Iraq that dates to 8700 BC. By 5000 BC, there aresigns of copper smelting, the refining of copper from simple copper compounds such asmalachite or azurite. Among archaeological sites in Anatolia, Catal Hoyuk (~6000 BC)features native copper artifacts and smelted lead beads, but no smelted copper. ButCan Hasan (~5000 BC) had access to smelted copper; this site has yielded the oldest knowncast copper artifact, a copper mace head.
Copper smelting appears to have been developed independently in several parts of theworld. In addition to its development in Anatolia by 5000 BC, it was developed inChina before 2800 BC, in the Andes around 2000 BC, in Central America around 600 AD, andin West Africa around 900 AD.
The use of bronze was so pervasive in a certain era of civilization that it has beennamed the-Bronze Age. The transitional period in certain regions between thepreceding Neolithic period and the Bronze Age is termed the Chalcolithic, with somehigh-purity copper tools being used alongside stone tools.
Copper has been mined for many centuries. By 2000 BC, Europe was using copper-tinalloys or ‘bronze’. The Bronze Age is taken as 2500 BC to 600 BC.
During the Bronze age, copper was mined in the British Isles in the followinglocations, among others: Great Orme in North Wales, Alderley Edge in Cheshire, theIsle of Man between England and Northern Ireland, Parys Mountain of Anglesey, and EctonMine in the Stafforshire Moorlands.
At Great Orme in North Wales, such working extended for a depth of 70 meters. AtAlderley Edge in Cheshire, carbon dates have established mining at around 2280 - 1890 BC(at 95% probability).
Copper mining in United States began with marginal workings by Native Americans andsome development by early Spaniards. Europeans were mining copper in Connecticut asearly as 1709. Westward movement also brought an expansion of copper exploitationwith developments of significant deposits in Michigan and Arizona during the 1850's andthen in Montana during the 1860's.
Copper was mined extensively in Michigan's Keweenaw Peninsula with the heart ofextraction at the productive Quincy Mine. Arizona had many notable depositsincluding the Copper Queen in Bisbee and the United Verde in Jerome. The Anaconda inButte, Montana became the nation's chief copper supplier by 1886.
Copper has also been mined at the Bingham Copper Mine near Salt Lake City, Utah as wellas mines in Nevada and Tennessee.
Copper is a reddish-colored metal, with a high electricaland thermal conductivity (silver is the only pure metal to have a higher electricalconductivity at room temperature). In oxidation copper is mildly basic. Copperhas its characteristic color because it reflects red and
Copper exists as a metallically bonded substance, allowing it tohave a wide variety of metallic properties.
Copper occupies the same family of the periodic table as silver and gold, since theyeach have one S-orbital electron on top of a filled shell. This similarity inelectron structure makes them similar in many characteristics. All have very highthermal and electrical conductivity, and all are malleable metals.
| 1s2 | ||
| 2s2 | 2p6 | |
| 3s2 | 3p6 | 3d10 |
| 4s1 |
In its liquid state, a clear copper surface without ambient light appears somewhat
Copper is insoluble in water (H2O) as well as in isopropanol.
Numerous copper alloys exist, many with important historical and contemporary uses. Speculum metal and bronze are alloys of copper and tin. Brass is an alloy ofcopper and zinc. Monel metal, also called cupronickel, is an alloy of copper andnickel. While the metal 'bronze' usually refers to copper-tin alloys, italso is a generic term for any alloy of copper, such as aluminum bronze, silicon bronze,and manganese bronze.
The purity of copper is expressed as 4N for 99.99% pure or 7N for 99.99999% pure. Thenumeral gives the number of nines after the decimal point when expressed as a decimal (eg4N means 0.9999, or 99.99%).
The main copper ore producing countries are Chile, United States, Indonesia, Australia,Peru, Russia, Canada, China, Poland, Kazakhstan, Zambia and Mexico.
Copper can be found as native copper in mineral form. Minerals such as thesulfides: chalcopyrite (CuFeS2), bornite (Cu5FeS4),covellite (CuS), chalcocite (Cu2S) are sources of copper, as are thecarbonates: asurite (Cu3(CO3)2(OH)2) andmalachite (Cu2CO3(OH)2) and the oxide: cuprite (Cu2O).
Most copper ore is mined or extracted as copper sulfides from large open pit mines inporphyry copper deposits that contain 0.4 to 1.0 percent copper. Examples include:Chuquicamata in Chile and El Chino Mine in New Mexico. The average abundance ofcopper found within crustal rocks is approximately 68 ppm by mass, and 22 ppm by atoms.
The Intergovernmental Council of Copper Exporting Countries (CIPEC), defunct since1992, once tried to play a similar role for copper as OPEC does for oil, but neverachieved the same influence, not least because the second-largest producer, the UnitedStates, was never a member. Formed in 1967, its principal members were Chile, Peru,Zaire, and Zambia.
The copper price has quintupled since 1999, rising from $0.60 per pound in June 1999 to$3.75 per pound in May 2006 where it began to drop steadily, most recently dropping toaround $2.20.
Copper is malleable and ductile, a good conductor of heat and, and, when very pure, agood conductor of electricity.
The two most familiar alloys of copper are bronze and brass. Bronze, the firstalloy created by people, is a mix of copper that contains as much as 25% tin. Early people used bronze to make tools, weaponry, containers and ornamental items. Brass,a mix of copper that contains between 5% and 45% zinc, was first used about 2,500 yearsago. The Romans were the first to make extensive use of brass, using it to make suchthings as coins, kettles and ornamental objects. Today, brass is also used in somemusical instruments, screws and other hardware that must resist corrosion.
It is used extensively, in products such as:
- Electronics:
- Copper wire.
- Electromagnets.
- Printed circuit boards.
- Electrical machines, especially electromagnetic motors and generators.
- Electrical relays, electrical busbars and electrical switches.
- Vacuum tubes, cathode ray tubes, and the magnetrons in microwave ovens.
- Wave guides for microwave radiation.
- Integrated circuits, increasingly replacing aluminum because of its superior electrical conductivity.
- As a material in the manufacture of computer heat sinks, as a result of its superior heat dissipation capacity to aluminum.
- Structural Engineering:
- The Statue of Liberty, for example, contains 179,220 pounds (81.3 tons) of copper.
- Alloyed with nickel, e.g. cupronickel and Monel, used as corrosive resistant materials in shipbuilding.
- Watt's steam engine.
- Household Products:
- Copper plumbing fittings and compression tubes.
- Doorknobs and other fixtures in houses.
- Roofing, guttering, and rainspouts on buildings.
- In cookware, such as frying pans.
- Most flatware (knoves, forks, spoons) contains some copper (nickel silver).
- Sterling silver, if it is to be used in dinnerware, must contain a few percent copper.
- Copper was sometimes used by the Inuit to make the cutting blade for ulus.
- Copper water heating cylinders
- Coinage:
- As a component of coins, often as cupronickel alloy.
- Euro coins contain different copper alloys
- Since 1982, U.S. Pennies are 0.8% copper by weight (Balance zinc 99.2%).
- U.S. Nickels are 75.0% copper by weight (Balance nickel 25.0%).
- Since 1965, U.S. Dimes and Quarters are 91.67% copper by weight (Balance nickel 8.33%).
- Biomedical:
- As a biostatic surface in hospitals, and to line parts of ships to protect against barnacles and mussels, originally used pure, but superseded by Muntz Metal. Bacteria will not grow on a copper surface because it is biostatic. Copper doorknobs are used by hospitals to reduce the transfer of disease, and Legionnaires' disease is suppressed by copper tubing in air-conditioning systems.
- Copper (II) sulfate is used as a fungicide and as algae control in domestic lakes and ponds. It is used in gardening powders and sprays to kill mildew.
- Copper-62-PTSM, a complex containing radioactive copper-62, is used as a Positron emission tomogaphy radiotracer for heart blood flow measurements.
- Chemical:
- Compounds, such as Fehling's solution, have applications in chemistry.
- As a component in ceramic glazes, and to color glass.
- Catalysis:
- Used in the Water gas shift reaction which converts carbon monoxide into carbon dioxide.
- Steam reforming which extracts hydrogen from hydrocarbons.
- Other Uses:
- Musical instruments, especially brass instruments and cymbals.
Copper is essential in all higher plants and animals. Copper is carried mostly inthe bloodstream on a plasma proten called ceruloplasmin. When copper is firstabsorbed in the gut it is transported to the liver bound to albumin. Copper is foundin a variety of enzymes, including the copper centers of cytochrome c oxidase and theenzyme superoxide dismutase (containing copper and zinc). In addition to itsenzymatic roles, copper is used for biological electron transport. The
Valence Electron Concentration Of Copper
Most mollusks and some arthropods such as the horseshoe crab use the copper-containingpigment hemocyanin rather than iron-containing hemoglobin for oxygen transport, so theirblood is blue when oxygenated rather than
It is believed that zinc and copper compete for absorption in the digestive tract sothat a diet that is excessive in one of these minerals may result in a deficiency in theother. The RDA for copper in normal healthy adults is 0.9 mgg/day. Because of its role infacilitating iron uptake, copper deficiency can often produce anemia like symptoms.
Hydrated copper sulfate (CuSO4·H2O), also known as blue vitrol,is the best known copper compound. It is used as an agricultural poison, as analgicide in water purification and as a blue pigment for inks. Cupric chloride (CuCl2),another copper compound, is used to fix dyes to fabrics. Cuprous chloride (CuCl) isa poisonous white powder that is chiefly used to absorb carbon dioxide (CO2).Copper cyanide (CuCN) is commonly used in electroplating.
Common oxidation states of copper include the less stable copper (I) state, Cu+;and the more stable copper (II) state, Cu2+, which forms blueor blue-green salts andsolutions. Under unusual conditions, a +3 state and even an extremely rare +4 statecan be obtained.
Copper (II) carbonate is green from which arises theunique appearance of copper-clad roofs or domes on some buildings. Copper (II) sulfateforms a blue crystalline pentahydrate which is perhaps themost familiar copper compound in the laboratory. It is used as a fungicide, known asBordeaux mixture.
There are two stable copper oxides, copper (II) oxide (CuO) and copper (I) oxide (Cu2O). Copper oxides are used to make yttrium barium copper oxide (YBa2Cu3O7-d)or YBCO which forms the basis of many unconventional superconductors.
- Copper (I) compounds : copper (I) chloride, copper (I) bromide, copper (I) iodide, copper (I) oxide.
- Copper (II) compounds : copper (II) carbonate, copper (II) chloride, copper (II) hydroxide, copper (II) nitrate, copper (II) oxide, copper (II) sulfate, copper (II) sulfide.
- Copper (III) compounds, rare: potassium hexafluorocuprate (K3CuF6)
- Copper (IV) compounds, extremely rare: cesium hexafluorocuprate (Cs2CuF6)
Copper (I) and Copper (II) can also be referred to by their common names cuprous andcupric.
Add aqueous sodium hydroxide. A blue precipitate ofcopper (II) hydroxide should form.
Ionic equation:
Cu2+(aq) + 2OH-(aq) Cu(OH)2(s)
The full equation shows that the reaction is due to hydroxide ions deprotonating thehexaaquacopper (II) complex:
[Cu(H2O)6]2+(aq) + 2OH-(aq) Cu(H2O)4(OH)2(s) + 2 H2O(l)
Adding aqueous ammonia causes the same precipitate to form. It then dissolves uponadding excess ammonia, to form a deep blue ammonia complex,tetraamminecopper (II).
Ionic equation:
Cu(H2O)4(OH)2(s) + 4NH3(aq) [Cu(H2O)2(NH3)4]2+(aq) + 4H2O (l)
A more delicate test than the ammonia is the ferrocyanide of potassium, which gives a
| Copper Ores | |
| Cuprite, CuO2 | Tenorite, CuO |
| Malachite, CuO3·Cu(OH)2 | Chalcocite, Cu2S |
| Covellite, CuS | Bornite, Cu6FeS4 |
| Azurite, Cu3(CO3)2(OH)2 | Chalcopyrite, CuFeS2 |
| chrysocolla, CuSiO3·2H2O | |
| Alternate Names | |
| Blue Vitriol, Copper Sulfate, CuSO4·5H2O | |
| Copper (I) Chloride, Cuprous Chloride, CuCl | |
| Copper (II) Chloride, Cupric Chloride, CuCl2 | |
Cuprous oxide, Cu2O is red, andcuprous sulphide, Cu2S is black. Both are very insoluble. In any solublecuprous compound, auto-oxidation generally occurs, 2Cu+ Cu + Cu++, producing thecupric salt. Cupric oxide, CuO is produced by heating copper in air, or by stronglyheating any oxygen-containing cupric salt. CuS is produced in an analogous way. In solution, the copper ion forms complex ions, such as Cu(H2O)4++,or Cu(NH3)4++. These are flat, square ions, of
Cupric sulphate, CuSO4·5H2O, called
The primary ores are sulphides and oxides, usually mixed with iron. Chalcociteis Cu2S, chalcopyrite CuFeS2, covellite CuS, and borniteCu3FeS3. The oxides are cuprite Cu2O, tenoriteCuO, malachite CuCO3·Cu(OH)2 (mineral verdigris), azurite2CuCO3·Cu(OH)2, and the silicate chrysocolla CuSiO3·2H2O.Many of these minerals make attractive specimens, because of their greenand blue colors, and can be carved into ornaments. They arenot seen as crystals or massive forms in the ore mined today, but widely disseminated inan earthy matrix.
There are two stable isotopes, 63Cu and 65Cu, along with a coupledozen radioisotopes. The vast majority of radioisotopes have half lives on the orderof minutes or less; the longest lived, 64Cu, has a half life of 12.7 hours,with two decay modes leading to two separate products.
| Isotope | Atomic Mass | Half-Life |
|---|---|---|
| 52Cu | 51.99718 | |
| 53Cu | 52.98555 | <300 ns |
| 54Cu | 53.97671 | <75 ns |
| 55Cu | 54.96605 | ~40 ms |
| 56Cu | 55.95856 | 93 ms |
| 57Cu | 56.949211 | 196.3 ms |
| 58Cu | 57.9445385 | 3.204 seconds |
| 59Cu | 58.9394980 | 81.5 seconds |
| 60Cu | 59.9373650 | 23.7 minutes |
| 61Cu | 60.9334578 | 3.333 hours |
| 62Cu | 61.932584 | 9.673 minutes |
| 63Cu | 62.9295975 | Stable |
| 64Cu | 63.9297642 | 12.700 hours |
| 65Cu | 64.9277895 | Stable |
| 66Cu | 65.9288688 | 5.120 minutes |
| 67Cu | 66.9277303 | 61.83 hours |
| 68Cu | 67.9296109 | 31.1 seconds |
| 69Cu | 68.9294293 | 2.85 minutes |
| 70Cu | 69.9323923 | 44.5 seconds |
| 71Cu | 70.9326768 | 19.4 seconds |
| 72Cu | 71.9358203 | 6.6 seconds |
| 73Cu | 72.936675 | 4.2 seconds |
| 74Cu | 73.939875 | 1.594 seconds |
| 75Cu | 74.94190 | 1.224 seconds |
| 76Cu | 75.945275 | 641 ms |
| 77Cu | 76.94785 | 469 ms |
| 78Cu | 77.95196 | 342 ms |
| 79Cu | 78.95456 | 188 ms |
| 80Cu | 79.96087 | ~100 ms |
| All copper compounds, unless otherwise known, should be treated as if they were toxic. Thirty grams of copper sulfate is potentially lethal in humans. The suggested safe level of copper in drinking water for humans varies depending on the source, but tends to be pegged at 1.5 to 2 mg/L. |
The DRI Tolerable Upper Intake Level for adults of dietary copper from all sources is10 mg/day. In toxicity, copper can inhibit the enzyme dihydrophil hydratase, anenzyme involved in haemopoisesis.
Symptoms of copper poisoning are very similar to those produced by arsenic. Coppery eructations and taste. Fatal cases are generally terminated by convulsions,palsy, and insensibility.
In cases of suspected copper poisoning, Ovalbumin is to be administered in either ofits forms which can be most readily obtained, as milk or whites of eggs. Vinegarshould not be given. The inflammatory symptoms are to be treated on generalprinciples, and so are the nervous.
A significant portion of the toxicity of copper comes from its ability to accept anddonate single electrons as it changes oxidation state. This catalyzes the productionof very reactive radical ions such as hydroxyl radical in a manner similar to fentonchemistry. This catalytic activity of copper is used by the enzymes that it isassociated with and is thus only toxic when unsequestered and unmediated. Thisincrease in unmediated reactive radicals is generally termed oxidative stress and is anactive area of research in a variety of diseases where copper may play an important butmore subtle role than in acute toxicity.
An inherited condition called Wilson's disease causes the body to retain copper, sinceit is not excreted by the liver into the bilel. This disease, if untreated, can leadto brain and liver damage. In addition, studies have found that people with mentalillnesses such as schizophrenia had heightened levels of copper in their systems. However it is unknown at this stage whether the copper contributes to the mental illness,whether the body attempts to store more copper in response to the illness, or whether thehigh levels of copper are the result of the mental illness.
Too much copper in water has also been found to damage marine life. The observedeffect of these higher concentrations on fish and other creatures is damage to gills,liver, kidneys, and the nervous system. It also interferes with the sense of smellin fish, thus preventing them from choosing good mates or finding their way to matingareas.
Number Of Valence Electrons Of Copper
| The metal, when powdered, is a fire hazard. At concentrations higher than 1 mg/L, copper can stain clothes and items washed in water. |
| Copper Data |
|
| Ionization Energy: 7.726 eV Estimated Crustal Abundance: 6.0×10-1 milligrams per kilogram Estimated Oceanic Abundance: 2.5×10-4 milligrams per liter |
| Transition Metals | ||||||||||
| Group | 3 (IIIB) | 4 (IVB) | 5 (VB) | 6 (VIB) | 7 (VIIB) | 8 (VIIIB) | 9 (VIIIB) | 10 (VIIIB) | 11 (IB) | 12 (IIB) |
| Period 4 | 21 Sc 44.95 | 22 Ti 47.86 | 23 V 50.94 | 24 Cr 51.99 | 25 Mn 54.93 | 26 Fe 55.84 | 27 Co 58.93 | 28 Ni 58.69 | 29 Cu 63.54 | 30 Zn 65.39 |
| Period 5 | 39 Y 88.90 | 40 Zr 91.22 | 41 Nb 92.90 | 42 Mo 95.94 | 43 Tc 98.00 | 44 Ru 101.0 | 45 Rh 102.9 | 46 Pd 106.4 | 47 Ag 107.8 | 48 Cd 112.4 |
| Period 6 | 57 La 138.9 | 72 Hf 178.4 | 73 Ta 180.9 | 74 W 183.8 | 75 Re 186.2 | 76 Os 190.2 | 77 Ir 192.2 | 78 Pt 195.0 | 79 Au 196.9 | 80 Hg 200.5 |
| Period 7 | 89 Ac 227.0 | 104 Rf 261.0 | 105 Db 262.0 | 106 Sg 266.0 | 107 Bh 264.0 | 108 Hs 269.0 | 109 Mt 268.0 | 110 Ds 269.0 | 111 Rg 272.0 | 112 Uub 277.0 |
